Reaction Intermediates
Reaction Intermediates: Overview
This topic covers concepts, such as, Reaction Intermediates, Carbanions, Carbene & Benzyne etc.
Important Questions on Reaction Intermediates
Correct order of rate hydrolysis or rate of reaction towards AgNO3 for following compounds is:
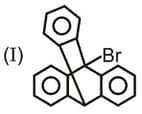
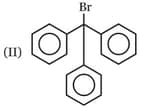
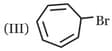
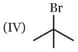
Which one of the following carbonyl compound when treated with dilute acid forms the more stable carbocation?
Write correct order of stability of following carbocations :
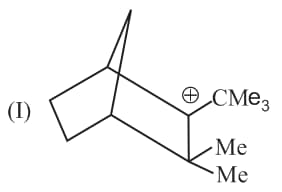
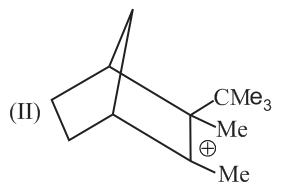
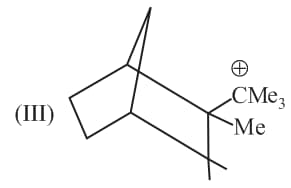
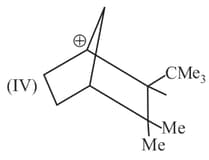
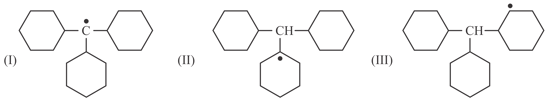
Rank the following free radicals in order of decreasing stability:
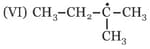
The correct stability order for the following species is
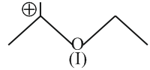
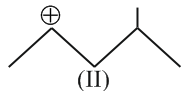
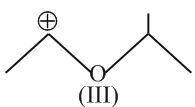
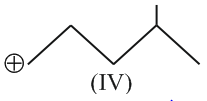
The correct order of the stability of carbocation is:
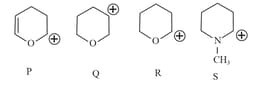
Compound from the following that will not produce precipitate on reaction with is
The decreasing order of hydride affinity for following carbocations is:
(a) 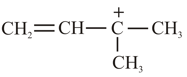
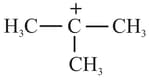
(2) 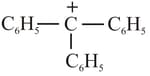
(3) 
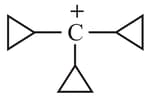
(4) 
Choose the correct answer from the options given below:
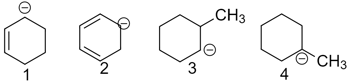
Arrange the following according to their stability
Which of the following carbanion is more stable?
Arrange the following in decreasing order of their stability.
Why vinyl carbanion is more stable than methyl carbanion
Which of the following is correct order of stability of given radicals
Arrange the following carbanions in correct order of stability
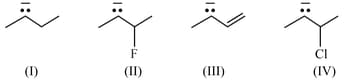
The most acidic hydrogen among given compounds is:
Which of the following carbocation changes to a more stable carbocation
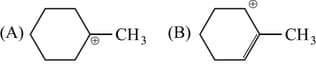
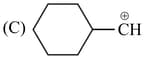
Which of the following is most stable cation?
An organic reaction occurs through making and breaking of bonds. The breaking of bonds may occur either homolytically leading to the formation of radicals or heterolytically generating positively and negatively charged species. The neutral species (free radicals, carbenes, nitrenes, etc.) and positively charged species being electron deficient are collectively called electrophiles while neutral and negatively charged species which are electron rich are called nucleophiles. An organic reaction usually involves the attack of a reagent (radicals, positively and negatively charged species) on the substrate molecule). The substrate molecule, although as a whole electrically neutral, has centers of low and high electron density due to displacement of bonding electrons. These electron displacements occur through inductive, electromeric, resonance and hyperconjugation effects. Whereas inductive effects involve displacement -electrons, resonance effects involve transfer of - and -electrons along a conjugated system. Hyperconjugatlon effects, on the other hand, involve --conjugation. Both inductive and hyperconjugation effects can be used to explain the stability of carbocations and free radicals which follow the stabilIty order : . The stability of carbanions, however, follows opposite order.
The stability of a molecule can be judged on the basis of contribution of its resonance structures. Resonance structures have same position of nuclei and have the same number of unpaired electrons. Among resonance structures, the one which has greater number of covalent bonds, has less separation of opposite charges, a negative charge on more electronegative and a positive charge on a more electropositive atom are more stable than others.
The geometry of a carbocation is _____.
_____ are formed by the homolytic cleavage of a covalent bond.
The stability of the free radicals due to inductive effect follows the order,
